Our mRNA platforms – revolutionizing vaccine technology
Our mRNA platforms are designed to activate or modulate the immune system from different angles, each providing a distinct strategy to address cancer or prevent infectious disease.
FixVac – off-the-shelf, indication-specific mRNA cancer vaccine platform
While every patient’s tumor has a unique composition, they can share certain sets of markers, so-called antigens. These markers are consistent within specific cancer types and are often expressed in many patients with the same type of cancer but not found in healthy cells in the body. As cancer cells have developed mechanisms to avoid detection by the immune system, a big challenge in treating cancer successfully is that the immune system does not recognize the cancer cells as malignant cells and thus does not trigger an immune response to attack and defeat this enemy. By presenting the right set of antigens for each cancer indication to the immune system, we can activate immune cells that recognize cancer-specific antigens and turn them against the cancer cells. This idea is the basis for our FixVac mRNA cancer vaccine platform.
The architecture of our FixVac platform
Our FixVac (Fixed Vaccine) platform candidates consist of a fixed combination of mRNA-encoded non-mutated tumor antigens, which are known to frequently express within specific cancer types. The mRNA is formulated with our proprietary RNA-lipoplex delivery formulation which is designed to enhance mRNA stability in the body as well as to target antigen-presenting dendritic cells (DCs) – the boot camps of our immune system. By enhancing the presentation of these specific tumor antigens by DCs to the immune system, our FixVac candidates aim to trigger a strong and precise innate and adaptive immune response against cancer cells expressing one or more of the respective tumor antigens. This approach offers the potential to also treat cancers with low mutational burden effectively, which represents half of all patients with metastatic melanoma.

We use optimized uridine mRNA (uRNA) for our FixVac candidates, which improves their immunostimulatory effect, aiming to boost vaccine efficacy – one of the major challenges currently seen in studies with tumor antigen-based cancer vaccines.
With this novel mRNA-based cancer vaccine approach, we aim to provide a highly tumor type specific, off-the-shelf therapy for cancer patients, particularly those with advanced, hard-to-treat disease.
At a glance: Our FixVac platform
Concept: Fixed combination of mRNA-encoded tumor antigens shared within specific cancer types aiming to trigger a strong and precise immune response against cancer cells expressing the respective antigen
mRNA format: Optimized uridine mRNA (uRNA) for improved vaccine efficacy
Therapeutic areas: Solid tumors, including melanoma, prostate cancer, HPV16+ head and neck cancer, ovarian cancer and non-small cell lung cancer
iNeST – individualized mRNA cancer vaccines
We are a pioneer and global leader in developing fully individualized immunotherapies that aim to address each patient’s unique tumor. With our Individualized Neoantigen Specific Immunotherapy (iNeST) platform, we have developed a first-in-kind, on-demand manufacturing process for mRNA vaccines designed to target mutations that are unique to the patient’s specific cancer, so-called tumor neoantigens.
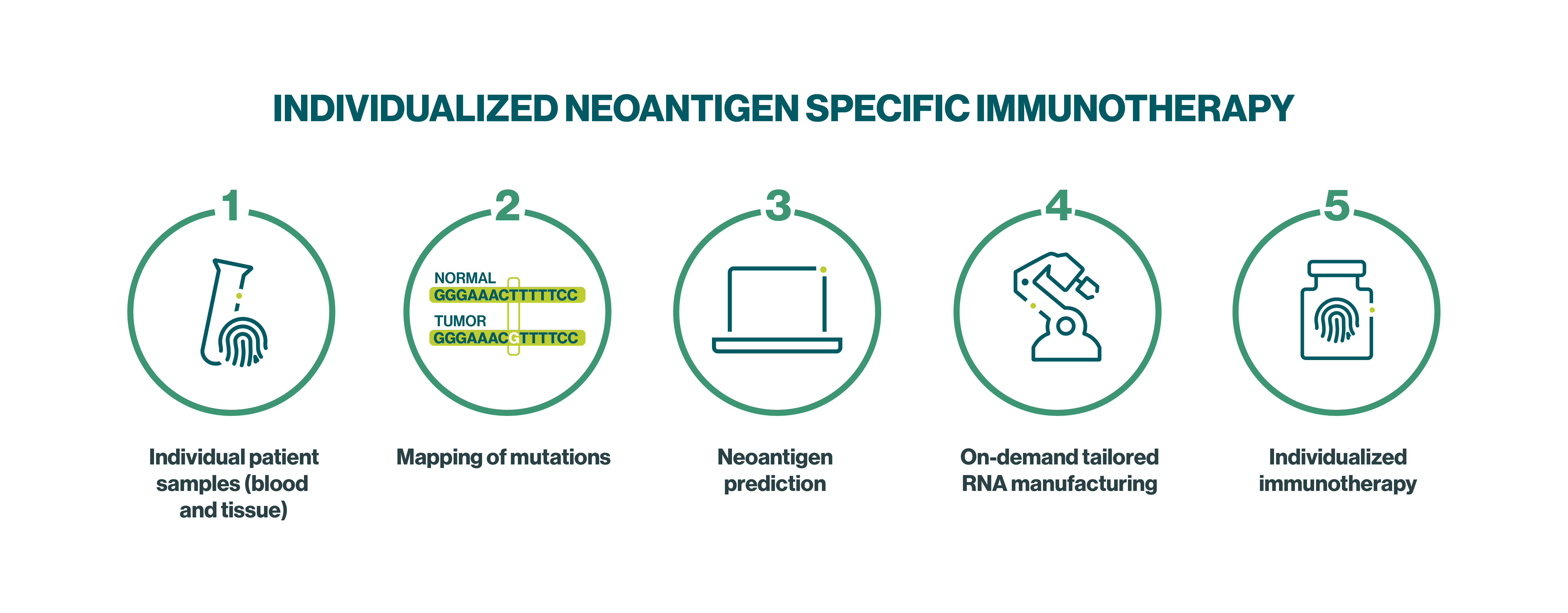
Our iNeST candidates contain mRNA encoding for an individually selected number of patient-specific tumor neoantigens aiming to induce a potent and precise immune response against the patient’s unique tumor.

We use optimized uridine mRNA (uRNA) for our iNeST candidates, which improves their immunostimulatory effect, aiming to boost vaccine efficacy – one of the major challenges currently seen in studies with tumor antigen-based cancer vaccines.
For more details on the iNeST manufacturing and treatment process see the fact sheet here.
At a glance: Our iNeST platform
Concept: Individualized mRNA cancer vaccine encoding for an individually selected number of patient-specific tumor neoantigens aiming to induce a potent and precise immune response against the patient’s unique tumor
mRNA format: Optimized uridine mRNA (uRNA) for improved vaccine efficacy
Therapeutic areas: Solid tumors, including melanoma and colorectal cancer
Infectious disease vaccine platform – transforming prophylactic vaccine technology
Our mRNA vaccines are designed to train the immune system to effectively detect and destroy a specific pathogen. They encode the building plan of a non-infectious piece of a pathogen, a so-called immunogen, and deliver it to antigen-presenting cells (APCs) in the body. There, the immunogen is produced and presented to the body’s immune cells.
With this approach, the immune cells learn how to recognize and fight the pathogen and can quickly react and eliminate the pathogen in case of a real infection. This quick reaction significantly reduces the risk of a pathogen-induced disease and transmission of the pathogen to other individuals.
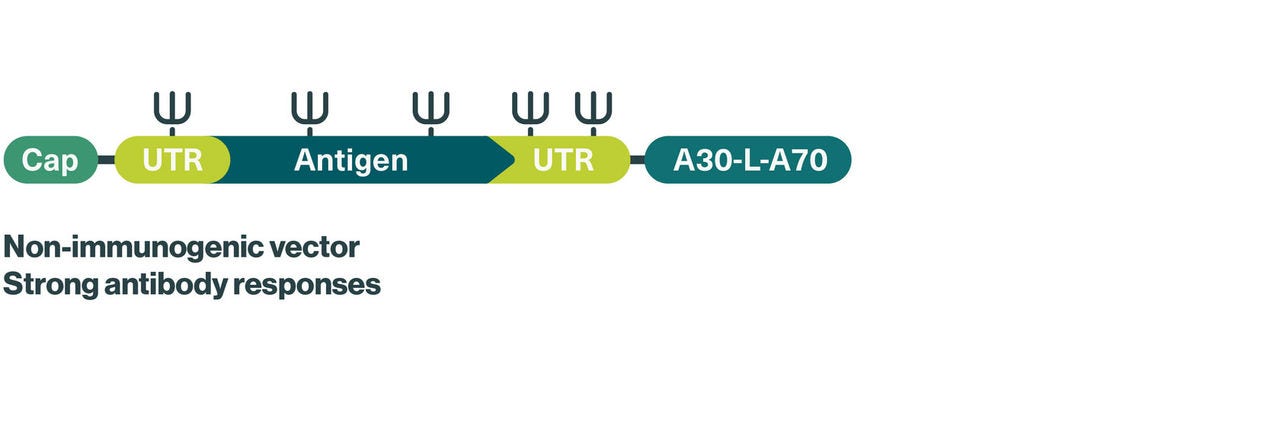
We use nucleoside-modified mRNA (modRNA) for our mRNA vaccine candidates, which contain naturally occurring modified building blocks conferring increased stability and reduced immunogenicity to minimize unwanted immune reactions and prolonging the duration of protein production – two major challenges in the development of safe and effective mRNA vaccines.
Our mRNA vaccine platform has brought forth the first commercially available COVID-19 vaccine, demonstrating the impact of our platform technology.
At a glance: Our mRNA Vaccine platform
Concept: mRNA vaccine encoding a single or multiple antigens to train the immune system to recognize and fight the respective pathogen in case of a real infection
mRNA format: Nucleoside-modified mRNA (modRNA) to minimize unwanted immune reactions and prolong the duration of protein production
Therapeutic areas: Infectious diseases, including COVID-19, seasonal influenza, malaria, HIV and tuberculosis
RiboCytokine – (re)activating the immune system
Cytokines are small proteins that play an important role in regulating the immune response against pathogens as well as cancer cells. Recombinant cytokines are currently used for the treatment of several infectious diseases and cancer indications, but their efficacy is hampered by their short half-life and low bioavailability upon application into the human body. Our RiboCytokine platform candidates aim to address these shortcomings by providing an mRNA-encoded blueprint plan to the patient’s body to produce immune-activating cytokines that support an anti-cancer immune response.

We use nucleoside-modified mRNA (modRNA) for our RiboCytokine candidates, which contain naturally occurring modified building blocks conferring increased stability and reduced immunogenicity to minimize unwanted immune reactions and prolonging the duration of protein production – two major challenges in the development of safe and effective mRNA-based therapeutics.
At a glance: Our RiboCytokine platform
Concept: Immune-activating cytokines encoded by mRNA and produced in the patient as an alternative to recombinant cytokines
mRNA format: Nucleoside-modified mRNA (modRNA) to minimize unwanted immune reactions and prolong the duration of protein production
Therapeutic areas: Solid tumors
Explore more

Pipeline

Publications
